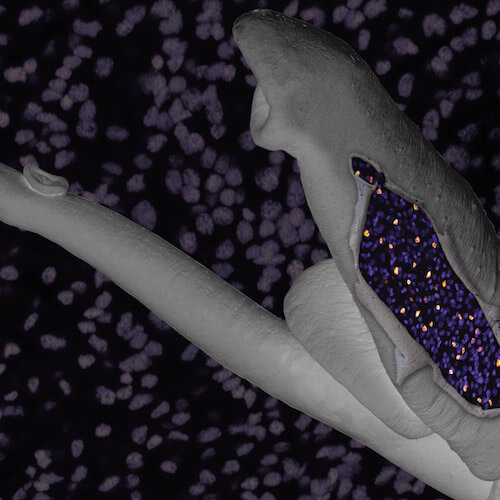
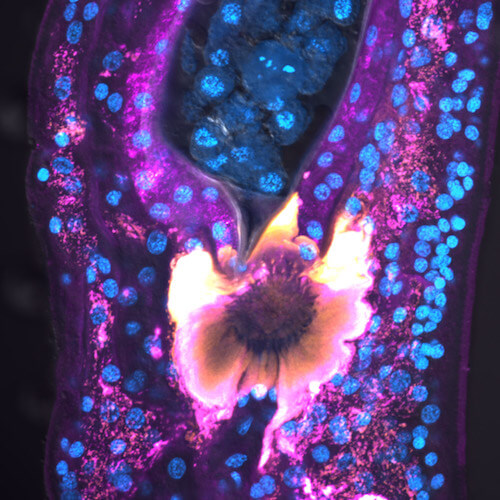
Zhao L, Wendt G.R., Collins J.J. III. 2024. A Krüppel-like factor establishes cellular heterogeneity during schistosome tegumental maintenance. PLoS Path.21(3):e1013002. [PDF]
•Preprint: bioRxiv
Wendt G.R. and Collins J.J. III. 2024. Horizontal gene transfer of a functional cki homolog in the human pathogen Schistosoma mansoni. bioRxiv
Issigonis M, Browder KL, Chen R, Collins J.J. III, Newmark PA. (2023) A niche-derived non-ribosomal peptide triggers planarian sexual development. Proc. Natl. Acad. Sci. U.S.A 121 (26) e2321349121
•Preprint: bioRxiv.
Wijshake T, Rose J III, Wang J, Marlar-Pavey M, Collins J.J. III, Agathocleous M. (2024) Schistosome infection impacts hematopoiesis. J Immunol. 212(4):607-616.
•Preprint: bioRxiv
Caldwell N, Afshar R, Baragaña B, Bustinduy AL, Caffrey CR, Collins JJ, Fusco D, Garba A, Gardner M, Gomes M, Hoffmann KF, Hsieh M, Lo NC, McNamara CW, Nono JK, Padalino G, Read KD, Roestenberg M, Spangenberg T, Specht S, Gilbert IH. 2023. Perspective on Schistosomiasis Drug Discovery: Highlights from a Schistosomiasis Drug Discovery Workshop at Wellcome Collection, London, September 2022. ACS Infect Dis. 9(5):1046-1055. [PDF]
Wendt G.R., Shiroor D.A., Adler C.E., Collins J.J. III. 2022. Convergent evolution of a genotoxic stress response in a parasite-specific p53 homolog. Proc. Natl. Acad. Sci. U.S.A 119 (37) e2205201119. [PDF]
Morales-Vicente DA, Zhao L, Silveira GO, Tahira AC, Amaral MS, Collins JJ III, Verjovski-Almeida S. 2022. Single-cell RNA-seq analyses show that long non-coding RNAs are conspicuously expressed in Schistosoma mansoni gamete and tegument progenitor cell populations. Front Genet 13:924877. [PDF]
Guzman M.A., Rugel A, Alwan S.N., Tarpley R, Taylor A.B., Chevalier F.D., Wendt G.R., Collins JJ III, Anderson T.J.C., McHardy S.F., LoVerde P.T. 2022. Schistosome Sulfotransferases: Mode of Action, Expression and Localization. Pharmaceutics. 14(7):1416. [PDF]
Chen R, Wang J, Gradinaru I, Vu H.S., Geboers S, Naidoo J, Ready J.M., Williams N.S., DeBerardinis R.J., Ross E.M., Collins J.J. III. 2022. A male-derived non-ribosomal peptide pheromone controls female schistosome development. Cell 185(9):1506-1520 [PDF]
Rozario T, Collins J.J. III, Newmark P.A. 2022. The good, the bad, and the ugly: From planarians to parasites. Current Topics in Developmental Biology 147:303-344. [PDF]
Romero A.A., Cobb S.A., Collins J.N.R., Kliewer S.A., Mangelsdorf D.J., Collins J.J. III. 2021. The Schistosoma mansoni nuclear receptor FTZ-F1 maintains esophageal gland function via transcriptional regulation of meg-8.3. PLoS Path. 17(12):e1010140. [PDF]
Dagenais M, Gerlach J.Q., Wendt G.R., Collins J.J. III, Atkinson L.E., Mousley A, Geary T.G., Long T. 2021. Analysis of Schistosoma mansoni Extracellular Vesicles Surface Glycans Reveals Potential Immune Evasion Mechanism and New Insights on Their Origins of Biogenesis. Pathogens. 29;10(11):1401.
Wendt G.R., Reese M.L., Collins J.J. III. 2021. SchistoCyte Atlas: A Single-Cell Transcriptome Resource for Adult Schistosomes. Trends Parasitol. 37(7):585-587. [PDF]
Perally S, Geyer K.K., Farani P.S.G., Chalmers I.W., Fernandez-Fuentes N, Maskell D.R., Hulme B.J., Forde-Thomas J, Phillips D, Farias L.P., Collins J.J. III, Hoffmann K.F. 2021. Schistosoma mansoni venom allergen-like protein 6 (SmVAL6) maintains tegumental barrier function. Int. J. Parasitol. (4):251-261.
Wang, J., Paz, C., Padalino, G., Coghlan, A., Lu, Z., Gradinaru, I., Collins, J. N. R., Berriman, M., Hoffmann, K. F., Collins, J. J. III. 2020. Large-scale RNAi screening uncovers therapeutic targets in the human parasite Schistosoma mansoni. Science 369:1649-1653 [PDF]
• Associated “PERSPECTIVE”: Anderson T.J.C., and Duraisingh, M.T. 2020. Transformative tools for parasitic flatworms. Science 369: 1562-1564.
•Preprint: bioRxiv
Wendt, G. R., Zhao, L., Chen, R., Lu, C., O’Donoghue, A. J., Caffrey, C. R., Reese., M.L., Collins, J. J. III. 2020. A single-cell RNAseq atlas of Schistosoma mansoni identifies a key regulator of blood feeding. Science 369:1644–1649 [PDF]
• Associated “PERSPECTIVE”: Anderson T.J.C., and Duraisingh, M.T. 2020. Transformative tools for parasitic flatworms. Science 369: 1562-1564.
•Preprint: bioRxiv
Wang, J, Chen, R, Collins J.J. III. 2019. Systematically improved in vitro culture conditions reveal new insights into the reproductive biology of the human parasite Schistosoma mansoni. PLoS Biol. 17(5):e3000254. [PDF]
•Preprint: bioRxiv
Wendt G.R., Collins J.N.R., Pei J, Pearson M, Bennett H.M., Loukas A, Berriman M, Grishin N.V., Collins J.J. III. 2018. Flatworm-specific transcriptional regulators promote the specification of tegumental progenitors in Schistosoma mansoni. eLife 7:e33221. [PDF]
• Associated “Insight”: Adler C.E. 2018. Tropical Disease: Dissecting the schistosome cloak. eLife 7:e36813.
Protasio AV, van Dongen S, Collins J, Quintais L, Ribeiro DM, Sessler F, Hunt M, Rinaldi G, Collins JJ, Enright AJ, Berriman M. MiR-277/4989 regulate transcriptional landscape during juvenile to adult transition in the parasitic helminth Schistosoma mansoni. PLoS NTDs 11(5):e0005559. [PDF]
Collins J.J. III 2017. Platyhelminthes. Current Biology 27(7):R252-R256 [PDF]
Collins J.N., Collins J.J. III 2016. Tissue Degeneration following Loss of Schistosoma mansoni cbp1 Is Associated with Increased Stem Cell Proliferation and Parasite Death in vivo. PLoS Pathogens 12(11):e1005963. [PDF]
Collins J.N., Collins J.J. III 2017. Methods for Studying the Germline of the Human Parasite Schistosoma mansoni. Methods Mol Biol. 1463:35-47. [PDF]
Wendt, G.W., Collins J.J. III 2016. Schistosomiasis as a disease of stem cells. Curr Opin Genet Dev.1463:35-47. [PDF]
Collins, J.J., III, Wendt, G.W., Iyer, H.I., Newmark, P.A. 2016. Stem cell progeny contribute to the schistosome host-parasite interface. eLife 5:e12473. [PDF]
• Associated “Insight”: Pearson MS, Loukas A. 2016. Stem cells: The parasite’s new clothes. eLife 5:e15957.
• Jim rappin' with Chris Smith for the eLife podcast
Wang, J, Collins, J.J. III. 2016. Identification of new markers for the Schistosoma mansoni vitelline lineage. Int J Parasitol.46(7):405-10. [PDF]
Lambrus, B.G., Cochet-Escartin, O, Gao, J, Newmark. P.A., Collins, E.M., Collins, J.J., III. 2015. Tryptophan hydroxylase Is Required for Eye Melanogenesis in the Planarian Schmidtea mediterranea. PLoS One. 2015 May 27;10(5):e0127074. [PDF]
Tharp, M. , Collins, J.J., III, Newmark, P.A. 2014. A lophotrochozoan-specific nuclear hormone receptor is required for reproductive system development in the planarian. Developmental Biology. 396(1):150-7. [PDF]
Collins, J.J., III, and Newmark, P.A. 2013. It's no fluke: Planarians as a model to understand schistosomes. PLoS Pathogens 9(7): e1003396. [PDF]
Collins, J.J., III, Wang, B., Lambrus, B.G., Tharp, M., Iyer, H., Newmark, P.A. 2013. Adult somatic stem cells in the human parasite Schistosoma mansoni.. Nature 494(7438):476-9. [PDF]
• Associated News and Views: Pearce, E.J., 2013. Parasitology: Rejuvenation through stem cells. Nature 494(7438):438-9.
• Featured on Carl Zimmer's Blog Phenomena: THE LOOM
Wang, B., Collins, J.J., III, and Newmark, P.A. 2013. Functional genomic characterization of neoblast-like stem cells in larval Schistosoma mansoni.. eLIFE 2:e00768. [PDF]
• Associated “Insight”: Sánchez Alvarado, A. 2013. Parasitism: On the trail of a tropical disease. eLIFE 2:e00768.
Chong, T., Collins, J.J., III, Brubacher J.L., Zarkower D, Newmark, P.A., 2013. A sex-specific transcription factor controls male identity in a simultaneous hermaphrodite. Nature Communications 4:1814. [PDF]
Collins, J.J., III*, King, R.S.*, Cogswell, A., Williams, D.L., Newmark, P.A. 2011. An atlas for Schistosoma mansoni. organs and life-cycle stages using cell type-specific markers and confocal microscopy. PLoS Negl Trop Dis. 5: e1009. [PDF]
Cogswell, A.A., Collins, J.J., III, Newmark, P.A., Williams, D.L. 2011. Whole mount in situ hybridization methodology for Schistosoma mansoni. Mol Biochem Parasitol. 178: 46-50. [PDF]
Collins, J. J., III, Hou, X., Romanova, E. V., Lambrus, B.G., Miller, C. M., Saberi, A., Sweedler, J.V., Newmark, P. A., 2010. Genome-Wide Analyses Reveal a Role for Peptide Hormones in Planarian Germline Development. PLoS Biology 8: e1000509. [PDF]